Natural Gas Calculation - Helmholtz Energy
Symbols
Thermodynamic Properties
Functional Forms
The Helmholtz free energy is a thermodynamic potential that measures the »useful« work obtainable from a closed thermodynamic system at a constant temperature and volume. The Helmholtz energy is defined as
f ≡ u - T·s (1)
All single-phase thermodynamic properties can be calculated as derivatives of the Helmholtz energy, as a function of temperature and density.
f(ρ,T) = f0(ρ,T) + fr(ρ,T) (2)
The dimensionless Helmholtz energy Φ uses independent variables of dimensionless density and temperature.
Φ(δ,τ) = Φ0(δ,τ) + Φr(δ,τ) (3)
For a certain composition of a mixture the ideal Helmholtz energy is
f0 = h0 - RT - Ts0 = T∫T0cp0dT + h00 - RT - T[T∫T0cp0TdT - Rlnρρ0 - RlnTT0 + s00 - Rn∑i=1xi·lnxi] (4)
Φ0 = -ττ∫τ0cp0R·τ2dτ + h00Rτ - 1 + τ∫τ0cp0R·τdτ + lnδδ0 + lnτ0τ - s00R + n∑i=1xi·lnxi (5)
The ideal gas part as well as the real fluid behavior is often described using empirical models.
Symbols
Natural Gas Calculation - Thermodynamic Properties
The functions for calculating compressibility factor, internal energy, enthalpy, entropy, heat capacity, speed of sound and other caloric properties are all related to the Helmholtz free energy and its derivates.
Compression Factor
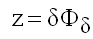 (9.18)
(9.18)
Internal Energy
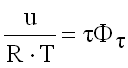 (9.19)
(9.19)
Enthalpy
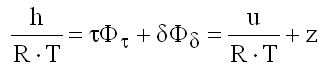 (9.20)
(9.20)
Entropy
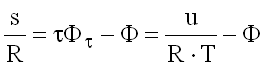 (9.21)
(9.21)
Isochoric Heat Capacity
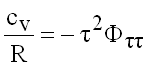 (9.22)
(9.22)
Isobaric Heat Capacity
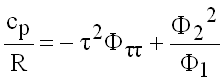 (9.23)
(9.23)
Joule Thomson Coefficient
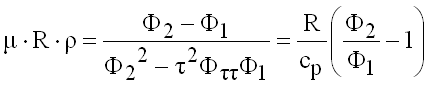 (9.24)
(9.24)
Isentropic Exponent
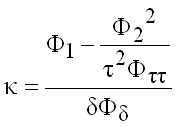 (9.25)
(9.25)
Speed of Sound
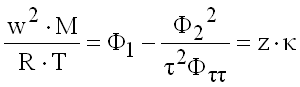 (9.26)
(9.26)
with
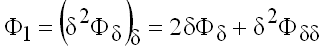 (9.27)
(9.27)
and
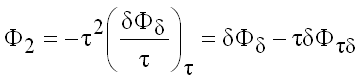 (9.28)
(9.28)
Functional Forms
The common functional forms of the fundamental equations are
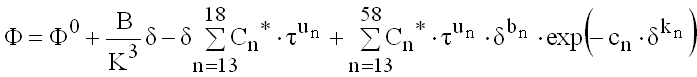 (9.29)
(9.29)
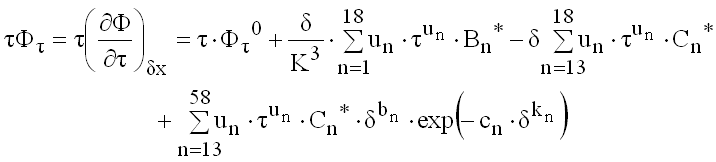 (9.30)
(9.30)
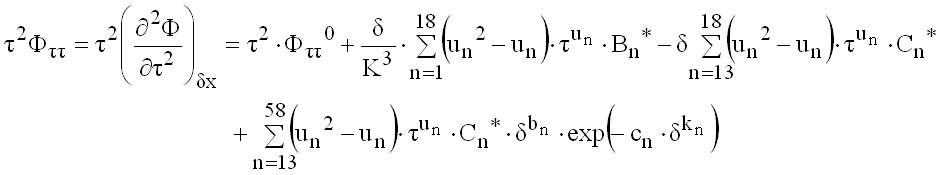 (9.31)
(9.31)
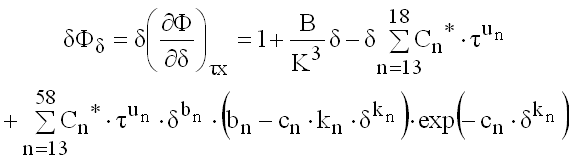 (9.32)
(9.32)
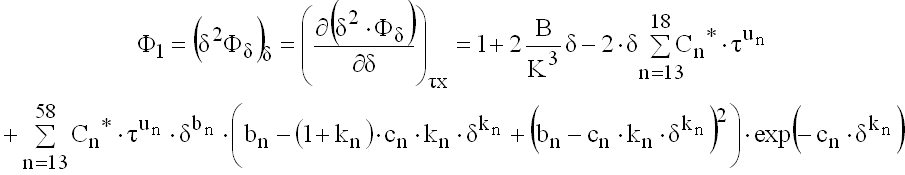 (9.33)
(9.33)
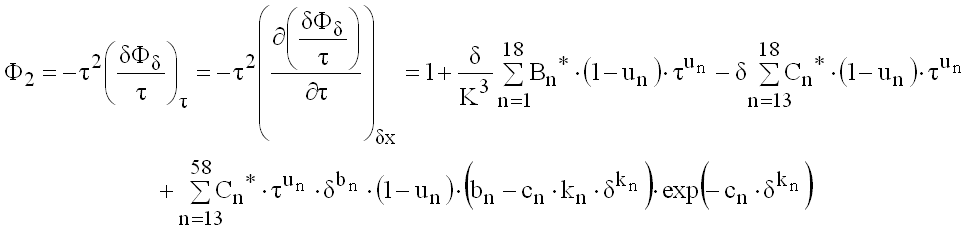 (9.34)
(9.34)
The various constants are given in the detailed documentation for the equation of state in annex D of ISO 20765-1.
